7,168,656
Title: Lightweight helicopter
Assignee: Council of Scientific and Industrial Research
Inventor: Bhaskar R. Pai
7,169,191
Title: Process for preparing a synthetic aluminum tanning agent
Assignee: Council of Scientific and Industrial Research
Inventor: Mookandi Kanthimathi; Palanisamy Thanikaivelan; Jonnalagadda Raghava Rao; Balachandran Unni Nair; Thirumalachari Ramasami
7,169,793
Title: Process for preparation of optically pure or optically enriched sulfoxide compounds, including amorphous esomeprazole and salts thereof
Inventor: Manne Satyanarayana Reddy; Muppa Kishore Kumar; Kikkuru Srirami Reddy; Koilkonda Purandhar; Keshaboina Sreenath
Assignee: Dr. Reddy’s Laboratories Limited
7,169,889
Title: Insulin prodrugs hydrolysable in vivo to yield peglylated insulin
Inventor: Nnochiri N. Ekwuribe; Muthukumar Ramaswamy; Jayanthi Rajagopalan
Assignee: Biocon Limited
Wednesday, January 31, 2007
Tuesday, January 30, 2007
Thailand Dilutes Two More Patent Monopoly
Thailand Ministry of Public Health has diluted Sanofi’s patent monopoly for anti-platelet drug --- Clopidogrel bisulphate and also Abbott’s patent monopoly for second-line anti-retroviral drug --- Lopinavir and ritonavir by issuing compulsory license which will allow Thailand to import and/or local production of generic versions. Earlier in December 2006, Merck’s Efavirenz was issued compulsory license by Thailand Government.
Monday, January 29, 2007
Glivec Update: Court Scheduled Final hearing in Next Month
A division bench of the Chennai High Court has scheduled final hearing in next month over Novartis petition challenging the constitutional validity of section 3(d) of the Patents Act, 1970 and decision of the Indian Patent Office to refuse grant of patent to Novartis patent application for crystalline form of Imatinib mesylate. Justice R. Balasubramaniam of the Chennai High Court today accepted and allowed Indian Pharmaceutical Alliance, Indian Generic Manufacturer and Sun Pharmaceuticals application for impleadment. During the hearing today, Novartis wanted to place the Mashelkar Committee Report on record which was opposed by the Indian Government. Novartis also wanted to convert its writ petition filed under Article 226 of the Indian Constitution to an appeal, which was further opposed by the Indian Government and Civil Society Groups.
Apotex Seeking Declaratory Judgment against GSK
Apotex has recently filed a complaint against GlaxoSmithKline in the U.S. District Court for the Eastern District of Virginia, Norfolk Division seeking declaratory judgment for non-infringement of Orange Book listed U.S. Patent # 5,068,249 (the ‘249 patent) covering oral syrup composition of ranitidine stabilized by adding effective amount of ethanol to the formulation. Apotex is seeking USFDA marketing approval for generic Zantac syrup and want the court to rule that its generic version won’t infringe the ‘249 patent that expires Mid-2009. Apotex in its complaint stated that it “faces potentially enormous infringement liability if it markets its generic product prior to the expiration” of the ‘249 patent and further added that “only a declaratory judgment from the court can alleviate this harm and allow Apotex to obtain approval of its product and compete in the lucrative ranitidine market free from infringement liability.”
The case is Apotex Inc. v. Glaxo Group Ltd., 07cv40, U.S. District Court for the Eastern District of Virginia, Norfolk Division.
Novartis continue to go forward with its Petition
A Division Bench at the Chennai High Court will be hearing Novartis petition today. In its petition, Novartis challenged ---
1. the constitutional validity of the section 3(d) of the Patents Act which considerably restricts grant of patents to existing drugs with improved therapeutic efficacy, and
2. a decision of the Indian Patent Office to refuse grant of patent to Novartis patent application for crystalline form of Imatinib Mesylate.
Novartis continue to go forward with its petition despite growing pressure from NGOs and public health groups across the world and also stated that it wanted the Indian Government to grant patent to its anti-cancer drug Glivec and ensure that Indian laws reflect international standards of patentability.
Thursday, January 25, 2007
Playing with the boys! Teva Sues Generic Rivals
Teva Pharmaceutical Industries Ltd. has filed patent infringement complaints against number of pharmaceutical companies which manufacture generic Zoloft (generically known as sertraline hydrochloride). Teva filed the complaints in the US District Courts of New Jersey, Delaware, Southern District New York, and Maryland for infringement of its U.S. Patent Nos. 6,600,073 (the ‘073 patent), 6,500,987 (the ‘987 patent), 6,495,721 (the ‘721 patent) and 6,897,340 (the ‘340 patent). Back in August, Teva launched generic Zoloft with 180-days marketing exclusivity after Pfizer lost its patent protection for sertraline hydrochloride in June last year. The exclusivity is due to end on February 6, 2007. In its complaint, Teva did not mentioned names of the companies or the number of firms it is suing.
Official Press Release.
Friday, January 19, 2007
Who Took Away The Better Half? Lipitor Patent Battle VII
Enforceability of the ‘995 patent
After analyzing legal principles of inequitable conduct, the Court concluded that Ranbaxy failed to established by clear and convincing evidence that the ‘995 patent was procured through inequitable conduct. In its arguments, Ranbaxy contended that Warner-Lambert withheld information concerning other patents in their portfolio and misrepresented the cholesterol inhibition activity of the compounds at issue. Specifically, Ranbaxy contended that the ‘080 patent and its CIP application were not disclosed to the PTO, and both these references give rise to prima facie unpatentability of the ‘995 patent based on obvious-type double patenting. The Court concluded that although the ‘080 patent was not revealed during the prosecution of the ‘995 patent, the Court is not persuaded that it was intentionally withheld. As for the data submission issue, the Court likewise concluded that it is not persuaded that the instances of non-disclosure cited by Ranbaxy are sufficient to demonstrate intent to deceive the PTO.
District Court Judgment
Judge Farnan Jr. is his memorandum opinion concluded: that (1) Ranbaxy’s ANDA product literally infringes the ‘893 and ‘995 patents; (2) that the ‘893 patent term extension is not invalid; and (3) that claim 6 is not invalid for failure to comply with section 112 paragraph 4; as anticipated or obvious; or for non-statutory double patenting; and (4) that the ‘995 patent is not unenforceable due to inequitable conduct.
Battle II Round II: Ranbaxy Appealed to CAFC
Following the District Court ruling, Ranbaxy filed an appeal with the U.S. Courts of Appeals for the Federal Circuit. CAFC, however, agreed with the District Court’s claim construction of claim 1 of the ‘893 patent and thus affirmed the District Court’s finding of infringement. And also affirmed the District Court’s ruling that the ‘893 patent term extension was not invalid.
With regard to the ‘995 patent, CAFC reverse the District Court’s ruling regarding the question of invalidity of the ‘893 patent under section 112 paragraph 4. Citing one of its recent judgments in Curtiss-Wright Flow Control Corp., 438 F. 3d 1374, 1380 (Fed. Circ. 2006), CAFC suggested that a violation of section 112, paragraph 4 renders a patent invalid just as violations of other paragraphs of section 112 would. The CAFC concluded that Ranbaxy correctly argued that claim 6 fails to “specify a further limitation of the subject-matter” of the claim to which it refers because it is completely outside the scope of claim 2. Accordingly, CAFC reversed the district court ruling with respect to the question of invalidity under section 112, paragraph 4 and invalidated claim 6 of the ‘995 patent for failure to comply with section 112, paragraph 4.
From the Desk of Patent Circle
It all started in 1995 when India became a founding member of the World Trade Organization (WTO) following the Uruguay Round of Multilateral Trade Negotiations including agreement on Trade-related Aspects of Intellectual Property Rights (TRIPS), and that was the beginning of India’s commitment (or rather obligation) to bring its patent law in uniformity to minimum requirements as negotiated (or rather stipulated) under TRIPS agreement. But the move to leverage patent law to TRIPS minimum requirements was not as simple and straight as what it seems to be, drawing large wave of criticisms from political parties, NGOs, Industries, and public-health activities. In 1998, India was forced to bring Mail-box provision for pharmaceutical and agricultural chemical products following complaints brought by the US and European Communities within the WTO Dispute Settlement Mechanism as a result of violation of Articles 27, 65 and 70 of the TRIPS agreement.
However, en-route to TRIPS obligation, India brought three amendments to existing patent law within a span of 10 years transitional period which finally ended on 31.12.2004. The first amended was passed in 1999 which brought provisions for Mail-box applications and Exclusive Marketing Rights (EMR) followed by second amendment in 2002 which along with important amendments amended definition of ‘invention’ and brought patent term in uniformity to TRIPS and also inserted provisions for Patent Cooperation Treaty (PCT). The third amendment brought in 2005 deleted provisions for Mail-box applications and EMR and introduced product patent protection in the field of pharmaceutical, chemical, biotechnology, and agro-chemical products. But the time gap between the second and third amendment concerned lots of public debates against the introduction of product patent in health-care sectors and also found Indian pharmaceutical sector divided on patent frontier, with a section of industry supporting product patent and other fearing abuse of product patent protection.
The 2005 amendment amended section 3(d) to exclude the possibility of ‘ever-greening’ and also inserted definitional clause for ‘pharmaceutical substance’. But section 3(d) after amendment left many things unclear than clear, making scope of section 3(d) technically unexplained and legally vague. However, during parliamentary debate over the Patents (Amendment) Bill, 2005 issues regarding patentability of micro-organisms and definition of ‘pharmaceutical substance’ to mean “a new chemical entity (NCE)” or “new medical entity (NME)” were raised which Commerce and Industry Minister assured to refer to an Expert Committee for detailed examination. Accordingly, a Technical Expert Group on Patent Law Issues was set by the Government of India under the chairmanship of Dr. R.A. Mashelkar, Director General, Council of Scientific and Industrial Research to decide whether (1) it would be TRIPS compatible to limit the grant of patent for pharmaceutical substance to new chemical entity or to new medical entity involving one or more inventive steps; and (2) it would be TRIPS compatible to exclude micro-organism from patenting.
The Expert Group consisting of Dr. R.A. Mashelkar, Prof. Goverdhan Mehta, Prof. Asis Datta, Prof. N.R. Madhava Menon, and Prof. Moolchand Sharma adopted a consultative approach before making recommendations and conclusion to the Government of India. The Expert Group consulted people from industries, NGOs, intellectual property attorneys, etc. through written submissions, presentations, etc. and also acknowledged the need for access of affordable medicines to Indian people at large, encouraging innovation by Indian industry, its current capabilities in R&D, and balancing of India’s obligation under international agreements with the wider public interest.
On December 29, 2006 the Technical Expert Group of Patent Law Issues submitted its recommendations to the Government of India, concluding that granting patents only to NCEs or NMEs, thereby excluding modifications and variant of NCEs, and excluding micro-organisms per se from patenting would contravene the TRIPS provisions. The Expert Group further concluded that detailed guidelines should be formulated and rigorously used by the Indian Patent Office for examining (1) pharmaceutical patent applications to prevent the grant of frivolous patents and ‘ever-greening’; and (2) patent applications involving micro-organisms from the point of view of substantial human intervention and utility.
In concluding its recommendations regarding NCEs or NMEs, the Expert Group explicitly distinguishes ‘ever-greening’ from ‘incremental innovation’; argued about the consequences in restricting patentability just to NCEs or NMEs; and examined the current level and R&D innovations of Indian pharmaceutical industry. In case of patentability of micro-organisms, the Expert Group argued about rapid growth of Indian biotech industry and India’s advantage and needs to reap the due of benefits from its rich bio-resources.
Patent Circle gives thumbs-up to Dr. R.A. Mashelkar and team for quite pragmatic conclusion and unbiased recommendations. It is important for Indian industry to understand that incremental innovations are important for the keeping the pace of the development. Most of the time the discussions are always based around big market players like Ranbaxy and Dr. Reddy’s which are slowly becoming capable to invent new molecules but what about small players? Incremental innovations are very much necessary to keep small companies going in this globally competitive environment.
Thursday, January 18, 2007
Patent Updates: U.S. Patents
Below are cited the recent U.S. Patents issued by the USPTO on January 16, 2007 to Indian Enterprises.
U.S. Patent No. 7,164,022
The ‘022 patent issued to Aurobindo Pharma Ltd. is directed to an improved process for the preparation of pure Finasteride of Formula I which comprises converting dihydrofinasteride of Formula IV to finasteride through novel protected dihydrofinasteride of Formula V and protected finasteride intermediates. The process comprises protecting dihydrofinasteride of Formula IV with suitable protecting group selected from --- benzoyl, substituted benzoyl, benzyl, substituted benzyl or benzhydryl group --- in an organic solvent selected from the group consisting of --- hydrocarbons, halogenated hydrocarbons, ketones, esters, and mixtures thereof --- to obtain a compound of formula V, further treating protected dihydrofinasteride of formula V with a silylating agent followed by quinine derivative with or without acid catalyst to obtain protected finasteride of formula VI and finally deprotecting compound of formula VI in an acidic or basic conditions to obtain Finasteride of formula.
IPC Class: C07D 221/18 (20060101); C07D 221/04 (20060101)
U.S. Patent No. 7,164,023
The ‘023 patent issued to Wockhardt is directed to crystalline S-(-)-9-fluoro-6,7-dihydro-8-(4-hydroxypiperidin-1-yl)-5-methyl-1-oxo-1H,- 5H-benzo[i,j]quinolizine-2-carboxylic acid L-arginine salt tetrahydrate, a process for its preparation and pharmaceutical formulations incorporating it as the active ingredient for use in treating microbial infections. The process comprises heating a suspension of S-(-)-9-fluoro-6,7-dihydro-8-(4-hydroxypiperidin-1-yl)-5-methyl-1-oxo-1H, 5H-benzo[i,j]quinolizine-2-carboxylic acid L-arginine salt tetrahydrate in an organic solvent and water at 70-80 ºC to obtain a clear solution, cooling the solution to provide a crystalline substance which is further isolated by filtration or centrifugation to obtain the crystalline form of S-(-)-9-fluoro-6,7-dihydro-8-(4-hydroxypiperidin-1-yl)-5-methyl-1-oxo-1H, 5H-benzo[i,j]quinolizine-2-carboxylic acid L-arginine salt tetrahydrate at 30-35 ºC and finally air drying of the crystalline form of S-(-)-9-fluoro-6,7-dihydro-8-(4-hydroxypiperidin-1-yl)-5-methyl-1-oxo-1H,- 5H-benzo[i,j]quinolizine-2-carboxylic acid L-arginine salt tetrahydrate at a temperature between 30-35 ºC.
IPC Class: C07D 401/04 (20060101); C07D 401/14 (20060101)
U.S. Patent No. 7,164,305
The ‘305 patent issued to STMicroelectronics Pvt. Ltd. is directed to a high-voltage input buffer circuit including a first NMOS transistor having its source terminal connected to the input pin, its gate terminal connected to a first reference voltage and its drain terminal connected to a first output terminal; a second NMOS transistor having its gate terminal connected to said first reference voltage and its source terminal connected to said first output terminal; a first PMOS transistor having its gate terminal connected to the drain terminal of said second NMOS transistor, its drain terminal connected to a second reference voltage lower than said first reference voltage and its source terminal connected to a second output terminal; a second PMOS transistor having its drain terminal connected to the drain terminal of said second NMOS transistor, its source terminal connected to said second output terminal, and its gate terminal connected to a control voltage; and a third PMOS transistor having its drain terminal connected to said second output terminal, its source terminal connected to a supply voltage, and its gate terminal connected to said control voltage.
IPC Class: H03L 5/00 (20060101)
U.S. Patent No. 7,165,068
The ‘068 patent issued to Zycus Infotech Pvt. Ltd. is directed to a method for classification of electronic catalog entries at any level into one or more categories, comprising the steps of: training the catalog classification system in at least one language with the help of pre-classified training catalogs, classifying the said catalog entry into top most relevant categories in the said category hierarchy, assigning a confidence value to each of the said classified catalog entry, wherein a user of said method can configure the method to classify said catalog in a specified confidence range, and automated sampling of the said classified catalogs for quality assurance.
IPC Class: G06F 17/30 (20060101)
Tuesday, January 16, 2007
Patent Updates: 33rd Annual Report
According to 33rd Annual Report as published under Section 155 of the Patents Act 1970 (2004-2005), under Section 5(2) (also referred as Mail-box provision) altogether 8,926 patent applications were received, 3,770 of which were through PCT National Phase route. Further, out of the total of 8,926 patent applications 973 were in respect of agrochemicals and rest from drugs and pharmaceuticals.
The total number of patents granted in 2004-2005 was 1,911 which included 67 patents for Food, 192 to Drug or Medicine, 414 to Mechanical, 245 to Electrical, 573 to Chemical and 278 on General. Total 6,857 patents were in force on the 31st March 2005.
Patent Update: Mashelkar Report
Eagerly awaited Mashelkar report is out now. Click here to read Report of the Technical Expert Group on Patent Law Issue.
Read More.
Monday, January 15, 2007
Who Took Away The Better Half? Lipitor Patent Battle VI
Validity of the ‘995 patent
(1) Double-patenting: Ranbaxy asserted claims 12 and 14 of U.S. Patent # 5,003,080 (the ‘020 patent) against the validity of the ‘995 patent. With regard to claim 14 of the ‘080 patent, the Court concluded that claim 14 claims a process for producing a single compound atorvastatin lactone. Neither this process nor this compound are contemplated by claim 6 of the ‘995 patent, and Ranbaxy has not demonstrated by clear and convincing evidence that claim 6 of the ‘995 patent recites an obvious variation of that which is recited in claim 14 of the ‘080 patent. The Court further stated that the inventions described in claim 14 of the ‘080 patent and claim 6 of the ‘995 patent are patentably distinct, and therefore claim 6 of the ‘995 patent is not invalid for non-statutory double patenting in light of claim 14 of the ‘080 patent.
As for claim 12 of the ‘080 patent, the Court likewise concluded that claim 12 presents an invention which is patentably distinct from that which is claimed in claim 6 of the ‘995 patent. Claim 12 of the ‘080 patent recites a process capable of making a variety of compounds, while claim 6 of the ‘995 patent is directed to a single compound, atorvastatin calcium.
(2) Obviousness: Ranbaxy asserted the ‘893 patent as the relevant prior to be considered in the analysis of whether the ‘995 patent is obvious. Before making analysis, the Court first identified the level of person skilled in the art and the relevant prior art, and then consider the differences between the prior art and the claimed subject-matter. After carefully analyzing, the Court concluded that Ranbaxy has not demonstrated that claim 6 of the ‘995 patent is obvious in light of the ‘893 patent as there was no motivation in the prior art to select the species compound of atorvastatin calcium from the genus of compounds identified in the ‘893 patent, and absent such a motivation, the Court cannot conclude that the ‘893 genus patent renders the ‘995 species patent obvious. Accordingly, the Court concluded that Ranbaxy has not demonstrated by clear and convincing evidence that claim 6 of the ‘995 patent is obvious.
(3) Anticipation: Ranbaxy contended that claim 6 of the ‘995 patent is anticipated by the ‘893 patent. Ranbaxy also pointed out that the ‘893 patent discloses calcium as one of the seven listed pharmaceutically acceptable salts. In response, Pfizer contended that prior art disclosure of a racemate does not anticipate either of its individual isomers. Pfizer also contended that the earlier disclosure of a genus does not anticipate an undisclosed species member of the genus. After applying the legal principles of anticipation, the Court, however, concluded that Ranbaxy has not established that the species compound claimed in the ‘995 patent is anticipated by the genus of compounds claimed in the ‘893 patent.
To be continued …
Alcon files Patent Lawsuit against Apotex
Alcon Laboratories has filed a patent infringement lawsuit in the U.S. District Court Southern District of Indiana (Indianapolis Division) against the generic manufacture Apotex alleging infringement of the U.S. Patent No. 5,641,805 (the ‘805 patent). The lawsuit is in response to Apotex’s notification to Alcon dated October 02, 2006 regarding Para IV certification for Patanol ophthalmic solution.
Earlier Apotex filed abbreviated new drug application (ANDA) No. 78-350 with the US FDA, seeking marketing approval for generic Patanol ophthalmic solution against its prior to the expiration of Orange Book listed ‘805 patent. Generically known as olopatadine hydrochloride, Patanol is approved for the treatment of the signs and symptoms of allergic conjunctivitis.
Ethicon Sued for Patent Infringement
Colorado-based Dentist, Dr. Stephen J. Smith, has filed a patent infringement lawsuit in the U.S. District Court for the District of Colorado against Ethicon, a Johnson & Johnson subsidiary, alleging infringement of the U.S. Patent No. 6,482,431 (the ‘431 patent). Dr. Smith also asserted claims for quantum meruit, unjust enrichment, breach of contract and promissory estoppel. The complaint was filed on November 27, 2006.
Saturday, January 13, 2007
Indian Engineer to Trigger First-to-Invent Objection against U.S. Patent
Srinivasan Gopalalkrishnan, an Indian Engineer recently objected that USPTO has issued a U.S. patent of a technology developed by him to another inventor. He claims to have developed a special fuel synthesizer for which Indian (IN200286) and British patent (GB2397782) are already been secured by him. He further added that he had filed PCT application (WO Publication WO03076790) in 2002. He is already in the process of procuring equivalent US application (US20040245085) via PCT national phase. He said, “However, the US Patent & Trademark Office has granted patent to a US inventor on August 22, 2006 though I filed for a PCT national phase patent in the US on April 30, 2004.”
Accordingly, Srinivasan has moved to Indian Innovators’ Association. However, to prove his just Inventorship, Srinivasan need to trigger First-to-Invent objection, needing to prove that he is first to conceive the invention and subsequently to reduce it to practice. Such objection would require USPTO to institute an interference proceeding between Srinivasan and US inventor.
AstraZeneca and Bristol-Myer Squibb signed US $ 1.35 billion diabetes deal
AstraZeneca has entered into US $ 1.35 billion agreement with Bristol-Myer Squibb to get access of Bristol’s two diabetes compounds --- Saxagliptin and Dapagliflozin currently in Phase III and Phase IIb development respectively. Under the terms of the alliance, the majority of development costs will be funded by AstraZeneca from 2007 through 2009.
Saxagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor disclosed in the U.S. Patent No. 6,395,767 (the ‘767 patent) whereas Dapagliflozin is a sodium-glucose cotransporter-2 inhibitor. Earlier, Bristol also entered into agreement with Otsuka Pharmaceutical Co. to develop and commercialize Saxagliptin in Japan.
Read more.
Read more.
Thursday, January 11, 2007
Innogenetics Wins Court Injunction Order, Abbott to Halt Sales of HCV products
The U.S. District Court for the Western District of Wisconsin has taken out a permanent injunction against Abbott Laboratories Inc. prohibiting Abbott from selling or using products, including components that infringe on Innogenetics’s patented Hepatitis C Virus (HCV) genotyping technology. The injunction also prohibits Abbott from exporting components of these products to foreign countries. During hearing, the court reiterated its finding that Innogenetics suffered irreparable harm from Abbott’s infringement. The court further found that Innogenetics has the capacity to serve the needs of the Hepatitis C diagnostic market and that Innogenetics had taken all reasonable steps to comply with GMP set out by the FDA. Innogenetics is reported to be evaluating the total case and its legal options including an appeal on willfulness.
Earlier in September 2005, Innogenetics sued Abbott alleging that Abbott was infringing the U.S. Patent No. 5,846,704 (the ‘704 patent) which covers a method of genotyping the Hepatitis C Virus. The court found the ‘704 patent was infringed as a matter of law. On September 01, 2006 a jury returned a unanimous verdict for Innogenetics that the ‘704 patent was valid in all respects. On September 08, 2006 same jury found Abbott had willfully infringed the ‘704 patent for a method of genotyping HCV and awarded Innogenetics US $ 7 million in damages. On January 04, 2007 the judge dismissed Abbott’s requests for a new trial, affirmed the jury’s finding that Abbott infringed the ‘704 patent, that the ‘704 patent was valid in all respects and approved the award of US $ 7 million in damages.
District Court Rejected Sun’s Motion for Summary Judgment
The U.S. District Court for the District of Maryland has rejected Sun Pharmaceutical’s motion for summary judgment of non-infringement of MedImmune’s U.S. Patent No. 5,591,731 (the ‘731 patent). At the same time, the court also granted Sun’s motion for summary judgment in part, ruling that Sun’s proposed amifostine product does not infringe MedImmune’s U.S. Patent No. 5,424,471 (the ‘471 patent).
The lawsuit was brought by MedImmune against Sun in August 2004 as a result of Para IV notification in June 2004 with respect to generic approval of anti-cancer product Ethyol, generically known as amifostine before expiry of Orange Book listed patents.
Wednesday, January 10, 2007
Who Took Away The Better Half? Lipitor Patent Battle V
The ‘995 patent
With regard to the ‘995 patent, claim construction dispute arises regarding claim 6 to cover the salt atorvastatin calcium. According to Ranbaxy, claim 6 cannot construed to cover the salt because claim depends on claim 2 and claim 2 narrows the subject-matter of claim 1 from atorvastatin acid or atorvastatin lactone, or pharmaceutically acceptable salts thereof. Ranbaxy argued that because claim 2 does not encompass salts, dependent claim 6 cannot cover the salt atorvastatin calcium. Ranbaxy specifically contended that a reading of claim 6 to include the salt would render the patent invalid under section 112, paragraph 4. In response, Pfizer contended that Ranbaxy’s claim construction is erroneous, because Ranbaxy incorrectly assumes that claim 6 must incorporate all of the limitation of claim 2.
The Court finds the language of claim 6 to be unambiguous to cover the salt, atorvastatin calcium and concluded that Court’s construction is consistent with the express language of the claim and the understanding of the claim language to one skilled in the art. The Court, however, recognizes that the Court’s claim construction conflicts with the requirements for dependent claims set forth in Section 112, paragraph 4 but concluded that this statutory provision provides no basis to invalidate a claim.
Based on above claim construction, the Court concluded that the Ranbaxy literally infringes claim 6 of the ‘995 patent.
Patent Term Extension
During trial Ranbaxy argued that the patent term extension granted for the ‘893 patent is invalid because (1) the ‘893 patent does not claim atorvastatin calcium; and (2) Pfizer voliated the duty of disclosure by knowingly withholding from the Director representations made by Warner-Lambert regarding the ‘893 patent during the prosecution of the ‘995 patent and its European Counterparts.
Based on the Court’s claim construction of the ‘893 patent, the Court concluded that Ranbaxy’s first argument provides no basis for invalidating the patent term extension of the ‘893 patent. With regard to Ranbaxy’s second argument, the Court concluded that information related to prosecution of the ‘995 patent and foreign counterparts of the ‘893 patent are irrelevant to a determination of the scope of the claims of the ‘893 patent and this concluded that Ranbaxy has not established that the alleged withheld information is material such that it was required to be disclosed during the application process for the patent term extension. Accordingly, the Court concluded that Ranbaxy has not established that the patent term extension of the ‘893 patent is invalid.
To be continued...
Who Took Away The Better Half? Lipitor Patent Battle
Who Took Away The Better Half? Lipitor Patent Battle II
Who Took Away The Better Half? Lipitor Patent Battle III
Who Took Away The Better Half? Lipitor Patent Battle IV
Monday, January 08, 2007
Dr. Reddy’s received a U.S. Patent for antiobesity and hypocholesterolemic compounds
Hyderabad-based Dr. Reddy’s Laboratories Limited has recently received a U.S. Patent No. 7,157,581 (the ‘581 patent) directed to novel b-aryl-a-oxysubstituted alkylcarboxylic acids of general formula I, their derivatives, their analogs, their taut omeric forms, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, and their pharmaceutically acceptable solvates useful for (1) reducing body weight and for the treatment and/or prophylaxis of diseases such as hypertension, coronary heart disease, atherosclerosis, stroke, peripheral vascular diseases and related disorders, (2) treating familial hypercholesterolemia, hypertriglyceridemia, lowering of atherogenic lipoproteins, VLDL (very low density lipoprotein) and LDL, (3) treating certain renal diseases including glomerulonephritis, glomerulosclerosis, nephrotic syndrome, hypertensive nephrosclerosis and nephropathy, (4) treating and/or prophylactic of insulin resistance (type II diabetes), leptin resistance, impaired glucose tolerance, dyslipidemia, disorders related to syndrome X such as hypertension, obesity, insulin resistance, coronary heart disease and other cardiovascular disorders, and (5) improving cognitive functions in dementia, treating diabetic complications, disorders related to endothelial cell activation, psoriasis, polycystic ovarian syndrome (PCOS), inflammatory bowel diseases, osteoporosis, myotonic dystrophy, pancreatitis, arteriosclerosis, retinopathy, xanthorna, inflammation and for the treatment of cancer. The ‘581 patent explicitly discloses list of 74 representative compounds covered within the structural scope of general formula I. Examples of the ‘581 patent in particularly exemplifies list of 30 compounds.
The ‘581 patent claims earliest priority from Indian Patent Application No. 2420/MAS/97 filed October 27, 1997. The ‘581 patent falls within the International classification C07D 239/02 (20060101) and the US classification 544/319 (Compounds wherein the chalcogen (i.e., oxygen, sulfur, selenium, or tellurium) is bonded directly at the 4- or 6-position of the six-membered hetero ring).
omeric forms, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, and their pharmaceutically acceptable solvates useful for (1) reducing body weight and for the treatment and/or prophylaxis of diseases such as hypertension, coronary heart disease, atherosclerosis, stroke, peripheral vascular diseases and related disorders, (2) treating familial hypercholesterolemia, hypertriglyceridemia, lowering of atherogenic lipoproteins, VLDL (very low density lipoprotein) and LDL, (3) treating certain renal diseases including glomerulonephritis, glomerulosclerosis, nephrotic syndrome, hypertensive nephrosclerosis and nephropathy, (4) treating and/or prophylactic of insulin resistance (type II diabetes), leptin resistance, impaired glucose tolerance, dyslipidemia, disorders related to syndrome X such as hypertension, obesity, insulin resistance, coronary heart disease and other cardiovascular disorders, and (5) improving cognitive functions in dementia, treating diabetic complications, disorders related to endothelial cell activation, psoriasis, polycystic ovarian syndrome (PCOS), inflammatory bowel diseases, osteoporosis, myotonic dystrophy, pancreatitis, arteriosclerosis, retinopathy, xanthorna, inflammation and for the treatment of cancer. The ‘581 patent explicitly discloses list of 74 representative compounds covered within the structural scope of general formula I. Examples of the ‘581 patent in particularly exemplifies list of 30 compounds.
The ‘581 patent claims earliest priority from Indian Patent Application No. 2420/MAS/97 filed October 27, 1997. The ‘581 patent falls within the International classification C07D 239/02 (20060101) and the US classification 544/319 (Compounds wherein the chalcogen (i.e., oxygen, sulfur, selenium, or tellurium) is bonded directly at the 4- or 6-position of the six-membered hetero ring).
 omeric forms, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, and their pharmaceutically acceptable solvates useful for (1) reducing body weight and for the treatment and/or prophylaxis of diseases such as hypertension, coronary heart disease, atherosclerosis, stroke, peripheral vascular diseases and related disorders, (2) treating familial hypercholesterolemia, hypertriglyceridemia, lowering of atherogenic lipoproteins, VLDL (very low density lipoprotein) and LDL, (3) treating certain renal diseases including glomerulonephritis, glomerulosclerosis, nephrotic syndrome, hypertensive nephrosclerosis and nephropathy, (4) treating and/or prophylactic of insulin resistance (type II diabetes), leptin resistance, impaired glucose tolerance, dyslipidemia, disorders related to syndrome X such as hypertension, obesity, insulin resistance, coronary heart disease and other cardiovascular disorders, and (5) improving cognitive functions in dementia, treating diabetic complications, disorders related to endothelial cell activation, psoriasis, polycystic ovarian syndrome (PCOS), inflammatory bowel diseases, osteoporosis, myotonic dystrophy, pancreatitis, arteriosclerosis, retinopathy, xanthorna, inflammation and for the treatment of cancer. The ‘581 patent explicitly discloses list of 74 representative compounds covered within the structural scope of general formula I. Examples of the ‘581 patent in particularly exemplifies list of 30 compounds.
The ‘581 patent claims earliest priority from Indian Patent Application No. 2420/MAS/97 filed October 27, 1997. The ‘581 patent falls within the International classification C07D 239/02 (20060101) and the US classification 544/319 (Compounds wherein the chalcogen (i.e., oxygen, sulfur, selenium, or tellurium) is bonded directly at the 4- or 6-position of the six-membered hetero ring).
omeric forms, their stereoisomers, their polymorphs, their pharmaceutically acceptable salts, and their pharmaceutically acceptable solvates useful for (1) reducing body weight and for the treatment and/or prophylaxis of diseases such as hypertension, coronary heart disease, atherosclerosis, stroke, peripheral vascular diseases and related disorders, (2) treating familial hypercholesterolemia, hypertriglyceridemia, lowering of atherogenic lipoproteins, VLDL (very low density lipoprotein) and LDL, (3) treating certain renal diseases including glomerulonephritis, glomerulosclerosis, nephrotic syndrome, hypertensive nephrosclerosis and nephropathy, (4) treating and/or prophylactic of insulin resistance (type II diabetes), leptin resistance, impaired glucose tolerance, dyslipidemia, disorders related to syndrome X such as hypertension, obesity, insulin resistance, coronary heart disease and other cardiovascular disorders, and (5) improving cognitive functions in dementia, treating diabetic complications, disorders related to endothelial cell activation, psoriasis, polycystic ovarian syndrome (PCOS), inflammatory bowel diseases, osteoporosis, myotonic dystrophy, pancreatitis, arteriosclerosis, retinopathy, xanthorna, inflammation and for the treatment of cancer. The ‘581 patent explicitly discloses list of 74 representative compounds covered within the structural scope of general formula I. Examples of the ‘581 patent in particularly exemplifies list of 30 compounds.
The ‘581 patent claims earliest priority from Indian Patent Application No. 2420/MAS/97 filed October 27, 1997. The ‘581 patent falls within the International classification C07D 239/02 (20060101) and the US classification 544/319 (Compounds wherein the chalcogen (i.e., oxygen, sulfur, selenium, or tellurium) is bonded directly at the 4- or 6-position of the six-membered hetero ring). Who Took Away The Better Half? Lipitor Patent Battle IV
Battle II Round I: US District Court
Pfizer sued Ranbaxy in response of Ranbaxy’s Abbreviated New Drug Application (ANDA) No. 76-477 filed with FDA, seeking marketing approval for generic bioequivalent of Atorvastatin Calcium. Pfizer filed patent infringement lawsuit in the U.S. District Court for the District Court of Delaware alleging infringement of U.S. Patent # 4,681,893 (the ‘893 patent) and U.S. Patent # 5,273,995 (the ‘995 patent) under 35 U.S.C. § 271 (e)(2). In particular, Pfizer alleges infringement of claims 1-4, 8 and 9 of the ‘893 patent and claim 6 of the ‘995 patent. In response, Ranbaxy counterclaimed Pfizer arguments alleging that it does not infringe either the ‘893 or ‘995 patents. Ranbaxy also challenged the validity of the patent term extension granted by the USPTO for the ‘893 patent. With regard to the ‘995 patent, Ranbaxy contented that the claim 6 of the ‘995 patent is invalid for double patenting, obviousness and anticipation. Ranbaxy also contended that the ‘995 patent is unenforceable as a result of inequitable conduct by Warner-Lambert Co. before the USPTO.
Judge Farnan Jr. in his Memorandum Opinion rendered the Court’s findings of fact and conclusion of law on the issues raised during the trial. In his Memorandum Opinion, the Judge carefully applied the legal principles of claim construction with respect to the ‘893 and ‘995 patents followed by applicable legal principles of infringement.
The ‘893 patent
With regard to the ‘893 patent, claim construction dispute arises regarding the structural formula I depicted by independent claim 1. According to Ranbaxy, the recited structural formula I represents only a genus of racemates whereas Pfizer contended that claim 1 represents racemates as well as R-trans enantiomers, S-trans enantiomers and unequal mixtures of R-trans and S-trans enantiomers. To support its claim construction, Ranbaxy contended that:
1. by common convention, a racemate can be represented by depicting one of its constituent enantiomers. But the court ruled out this argument stating that Ranbaxy has not demonstrated that, to one skilled in the art, such a depiction always or even usually specifies a racemate.
2. the reaction sequences and examples of the ‘893 patent are directed to racemate. But the court ruled out this argument stating that the ‘893 patent is not limited to its examples and provides no indication that it should be so limited.
3. if the Court accepts Pfizer’s construction of the ‘893 patent, the patent is invalid for lack of written description, because the patent does not disclose any methods for making enantiomers. But the Court ruled out this argument stating that the generic formula description contained in the ‘893 patent is sufficient to satisfy the written description requirement, regardless of whether the specific isomeric compounds are individually described in the patent.
4. Warner-Lambert’s representations during the prosecution of foreign counterparts to the ‘893 patent demonstrate that the ‘893 patent is limited to racemate. But the Court ruled out this argument stating that Warner-Lambert’s statements during the prosecution of foreign counterparts of the ‘893 patent are irrelevant to the Court’s claim construction of the ‘893 patent issued in the United States.
In sum, the Court concluded that its claim construction of the ‘893 patent is supported by the specification and the express language of the claim. The Court positioned that Ranbaxy’s claim construction is primarily based on extrinsic evidence, which is irrelevant to claim construction and inconsistent with the intrinsic evidence of the ‘893 patent. After considering the claim language, the specification and the prosecution history of the ‘893 patent, the Court concluded that the ‘893 patent is not limited to racemate and embrace all trans-form isomers, including enantinomeric atorvastatin calcium.
Based on above claim construction, the Court concluded that Ranbaxy’s ANDA product literally infringes claims 1-4 of the ‘893 patent. The Court also concluded that Ranbaxy is likely to market or sell a composition containing atorvastatin calcium as embraced by claim 8 of the ‘893 patent, for use in the method claimed by claim 9 of the ‘893 patent for inhibiting cholesterol biosynthesis in a patient in need of such treatment. Therefore, the Court concluded that Ranbaxy literally infringes claim 8 and 9 of the ‘893 patent.
To be continued...
Who Took Away The Better Half? Lipitor Patent Battle III
The ‘281 Patent
Ranbaxy in its second action challenged the ‘281 patent validity, particularly claim 1 covering hemicalcium salt of atorvastatin on the grounds of:
(1) anticipation by the WO Application, and
(2) obviousness in the light of the Application.
Here, yet again, Judge Pumfrey followed a much careful and meticulous approach while dealing with both the anticipation and obviousness issues.
Anticipation Issue
Before deciding anticipation issue, the court laid down the principles of anticipation and went on addressing the WO Application in great detail. The court acknowledged that the WO Application is directed for ways of making particularly, Formula (I) –
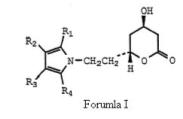 which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia.
which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia.
 which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia.
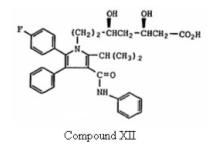
which is same of formula (I) of the ‘633 patent save that group X is explicitly ethyl, (-CH2CH2-) and more particularly directed to Formula Ia. The court further went on addressing the WO Application with particular emphasis on(1) Compound XII –
which is the ring-opened dihydroxy acid form of Formula Ia, and
(2) a statement from the WO Application stating that “the preferred isomer of this invention is the 4R, 6R-isomer of the compounds of Formula I, Ia and XII.”
The court also addressed a paragraph from the WO Application quoting –
‘In the ring-opened dihydroxy acid form, compounds of the present invention react to form salts with pharmaceutically acceptable metal and amine cations formed from organic and inorganic bases. The term "pharmaceutically acceptable metal salt" contemplates salts formed with the sodium, potassium, calcium, magnesium, aluminum, iron and zinc ions.'”
With this, the court followed that the material claimed in claim 1 is an expressly specified salt (calcium) of the preferred isomer of one of three materials explicitly specified. The court after carefully addressing the WO Application disclosure and the ‘281 patent, concluded that the final structure formula of the ‘281 patent and compound XII of the WO Application are identical, save that the ‘281 patent refers calcium salt whereas the WO Application refers the acid form. The court further concluded that the WO Application gives specific directions to make the three preferred enantiomers, and one of which falls within the claim 1 of the ‘281 patent, and thereby making a clear case of anticipation of claim 1 of the ‘281 patent.
Obviousness
While dealing obviousness issue, the dispute arises as to the right approach to obviousness in this case. Warner-Lambert took as their exemplar the decision of the Technical Board of Appeals of the European Patent Office on the present patent. On a problem-solution approach, the EPO Board of Appeal found the selection of the calcium salt and the particular enantiomer to be inventive. Judge Pumfrey found otherwise, expressing preference for the historical English approach in Windsurfing v. Tabur Marine [1985] RPC 59 (CA)) (the Windsurfer analysis) over the EPO’s problem-solution approach.
An EPO Board of Appeal in this case had been persuaded, during prosecution, to reverse an obviousness rejection on the basis that the selection of calcium from among previously described pharmaceutical metal salts (potassium, sodium, calcium, iron, aluminum, magnesium and zinc), together with selection of one of the two enantiomers from the racemic mixture, formed an inventive selection because this combination was said to improve the handling properties of the solid salt (particularly its hygroscopicity and solubility).
However, according to Judge Pumfrey, first there was no inventiveness in resolving the racemate into its enantiomers and selecting a particular enantiomer. On the evidence, there was no particular difficulty in performing resolution in this particular case.
Second, there was no inventiveness in selecting a particular salt, and least of all the calcium salt, from among the seven possibilities already disclosed. Once a pharmaceutically active component is isolated, conducting a salt screen is a standard procedure to identify the best salt for administration. Potassium, sodium and calcium salts are the most commonly used. Judge said, “We find ourselves in the strange position that if the sodium salt had been satisfactory, there would have been no invention in going for the calcium instead, but since it was not, there is an invention.”
The judge was critical of permitting the patentee to reformulate the problem on the basis of an advantage discovered after the priority date, or one known before the priority date to the patentee, but not disclosed by him in the patent application. In his own words, Judge Pumfrey stated “How can one solve an objective problem that one did not know existed?”
In his judgment, Judge Pumfrey refused to grant the declaration of non-infringement sought by Ranbaxy in respect of the ‘633 patent and invalidated the ‘281 patent for anticipation and obviousness.
Battle I Round II: U.K. Court of Appeal
Ranbaxy later appealed to United Kingdom’s Court of Appeal against the ruling of the U.K. High Court judgment regarding declaration of non-infringement in respect of the ‘633 patent. However, Court of Appeal affirmed the High Court ruling that Ranbaxy’s proposed generic product of Atorvastatin Calcium would infringe the ‘633 patent. The appeals court also affirmed High Court ruling regarding invalidity of the ‘281 patent.
To be continued...
Related Posts---
Who Took Away The Better Half? Lipitor Patent Battle
Who Took Away The Better Half? Lipitor Patent Battle II
Novartis Filed Complaint for Patent Infringement against Lupin
Novartis has filed a complaint for patent infringement following a notification from Lupin Pharmaceuticals that it has filed an abbreviated new drug application (ANDA) under Para IV certification with the US FDA seeking marketing approval for generic Lotrel before expiry of Orange Book listed patent. In its complaint, Novartis alleged that Lupin’s ANDA No. 78-466 would be infringing the U.S. Patent No. 6,162,802 (the ‘802 patent) and subsequently demanded the district court to permanently enjoin Lupin from making , using, offering to sell, or importing the Lupin’s generic Lotrel until after the expiration of the ‘802 patent (including six month additional pediatric exclusivity period).
Lotrel is a combination of amlodipine and benazepril hydrochloride, which is protected the ‘802 patent directed to a pharmaceutical composition consisting essentially of a range of ratios of specified amounts of benazepril and amlodipine (or pharmaceutically acceptable salts of either or both), as well as a method of treating condition selected from a group consisting of hypertension, in a human, consisting of administering a daily dose of a range of ratios of specified amounts of benazepril and amlodipine (or pharmaceutically acceptable salts of either or both). Lupin in its ANDA contended and certified that the ‘802 patent is invalid and/or will not be infringed by the Lupin proposed generic Lotrel product.
The complaint has been filed in the U.S. District Court for the District of New Jersey and the U.S. District Court for the District of Maryland (Northern Division).
Thursday, January 04, 2007
Lek filed Patent Lawsuit against BMS & Watson, seeking monetary compensation!
Novartis’s unit Lek has filed a patent infringement lawsuit in the U.S. District Court for the Eastern District of Texas (Marshall) against Bristol-Myer Squibb and Watson Laboratories Inc. seeking royalties against its two U.S. Patent Nos. 6,740,775 (the ‘775 patent) and 7,078,558 (the ‘558 patent) covering crystalline forms of pravastatin, the key ingredient of anti-cholesterol drug Pravachol. Lek claims that the BMS version of the drug uses crystalline forms of pravastatin covered by ‘775 and ‘558 patents, which claims sodium salt pravastatin in crystalline form having improved purity and stability. Pravastatin is exemplified and disclosed in the U.S. Patent No. 4,346,227 (the ‘227 patent) which recently expired on April 20, 2006. Watson markets a generic Pravachol under an agreement with BMS. Lek, which also received Food and Drug Administration approval to market a generic Pravachol in October 2006, is seeking monetary compensation and a court order to bar BMS and Watson from infringing the patents.
Lek DD v. Bristol-Myers Squibb Co., 2:06-cv- 00547, U.S. District Court, Eastern District of Texas (Marshall).
Wednesday, January 03, 2007
Latest Patents Issued by Indian Patent Office (Kolkata Branch)
Following patents are issued on and published in The Patent Office Journal dated December 29, 2006.
Indian Patent No. 200489 titled Heat recovery steam generator assigned to Alstom Power Inc. is issued against the Indian Patent Application no. IN/PCT/2000/305 dated March 23, 1999. Corresponding WO Publication no. WO9951916.
Indian Patent No. 200468 titled Method and device for reduction of charge consumption in mobile multimode communication units assigned to Siemens is issued against the Indian Patent Application no. 2416/CAL/1997 dated December 19, 1997. Corresponding U.S. Patent No. 6,400,961.
Indian Patent No. 200467 titled Method for determining an impact point of a fired projectile relative to the target assigned to Thales Nederland B.V. is issued against the Indian Patent Application no. 1646/CAL/1997 dated September 05, 1997. Corresponding U.S. Patent No. 6,037,896.
Indian Patent No. 200465 titled Device and method for diversity combining signals on common channel in CDMA communication system assigned to Samsung Electronics is issued against the Indian Patent Application no. 692/CAL/1999 dated August 05, 1999.
Indian Patent No. 200464 titled Corrosion inhibiting solutions and process for refrigeration systems assigned to Shyam Kumar Verma et al. is issued against the Indian Patent Application no. 2025/CAL/1997 dated October 26, 1997.
Indian Patent No. 200317 titled A servo error detecting apparatus for recording data and/or reproducing data from a disk assigned to Samsung Electronics is issued against the Indian Patent Application no. 171/KOL/2003 dated August 27, 2003.
Indian Patent No. 200243 titled Connector for a corrugated pipe with locking portion assigned to PMA AG is issued against the Indian Patent Application no. 45/CAL/2000 dated January 31, 2000.
Indian Patent No. 200242 titled A tire-grasping device for a post-vulcanization inflating device assigned to Ichimaru Giken Co. is issued against the Indian Patent Application no. IN/PCT/02/1465 dated January 09, 2001.
Indian Patent No. 200241 titled Coupling for rotationally connecting actuating shafts of weave machine and weaving looms assigned to Baruffaldi S.p.A. is issued against the Indian Patent Application no. 514/CAL/2001 dated September 07, 2001.
Indian Patent No. 200240 titled A tube on which yarn is wound to form a yarn stick assigned to Sonoco Development, Inc. is issued against the Indian Patent Application no. 00278/KOL/2003 dated May 19, 2003.
Indian Patent No. 200238 titled A process for coating a substrate assigned to IIT is issued against the Indian Patent Application no. 229/CAL/2001 dated April 17, 2001.
Indian Patent No. 200237 titled A process for making refractories for torpedo ladle assigned Tata Refractories Limited is issued against the Indian Patent Application no. 165/CAL/2001 dated March 23, 2001.
Indian Patent No. 200236 titled A rail vehicle with a modular vehicle body and a method for the production of such rail vehicle assigned to ABB Daimler-Benz, Siemens and Deutsche Waggonbau is issued against the Indian Patent Application no. 314/CAL/1997 dated February 20, 1997.
Indian Patent No. 200235 titled A process for preparing a decontaminating agent from the plant Moringa pterygosperma/Olifer a for decontaminating proteinacceous food products like meat, fish and egg assigned to Pravat Kumar Mukherjee et al. is issued against the Indian Patent Application no. 540/KOL/2003.
Indian Patent No. 200234 titled A collar for interconnecting one electrical conductor with another electrical conductor assigned to Eaton Corporation is issued against the Indian Patent Application no. 2089/CAL/1998 dated November 26, 1998.
Indian Patent No. 200233 titled A laundry appliance assigned to Fisher & Paykel Limited is issued against the Indian Patent Application no. IN/PCT/2001/00597 dated December 21, 1999.
Indian Patent No. 200226 titled Method of demonstrating unauthorized imitation of a device and safe guarding a package assigned to ABB Patent GmbH is issued against the Indian Patent Application no. 318/CAL/2000 dated June 01, 2000.
Indian Patent No. 200229 titled An isometric setsquare assigned to Padma Charankar is issued against the Indian Patent Application no. 76/CAL/1999 dated February 01, 1999.
Indian Patent No. 200228 titled A method for data transmission in a mobile radio system assigned to Siemens is issued against the Indian Patent Application no. IN/PCT/2001/00457 dated November 02, 1999.
Indian Patent No. 200227 titled Hand grippable combined keyboard and game controller assigned to Alpha Grip Inc. is issued against the Indian Patent Application no. 363/CAL/2002 dated June 11, 2002.
Indian Patent No. 200220 titled Apparatus for measuring bit error ratio by using a viterbi deoder assigned to Samsung Electronics is issued against the Indian Patent Application no. 1734/CAL/1997 dated September 25, 1997.
Above referred Indian patents are published under section 43(2) of the Patents Act, 1970 and will be open for post-grant opposition under section 25(2) for a period of one year from the date of issuance.
Tuesday, January 02, 2007
Latest U.S. Patents Issued to Indian Entities (Issued on December 26, 2006)
U.S. 7,153,317 titled Disposable guarded surgical scalpel assigned to Ribbel International Limited.
U.S. 7,153,528 titled Process for preparation of hypoglycemic foods and formulations thereof assigned to CSIR.
U.S. 7,153,806 titled Encapsulated oxo-bridged organometallic cluster catalyst and a process for the preparation thereof assigned to CSIR.
U.S. 7,153,983 titled Chemo-enzymatic process for the preparation of optically enriched b-benzyl-g-butyrolactones assigned to CSIR.
U.S. 7,154,299 titled Architecture for programmable logic device assigned to STMicroelectronics Pvt. Ltd.
U.S. 7,154,318 titled Input/output block with programmable hysteresis assigned STMicroelectronics Pvt. Ltd.
Subscribe to:
Posts (Atom)